Ice Liquid Water
What is the difference between a material in the solid form and that same material in a liquid form? Everyone is familiar with the difference between water and ice. General chemistry students may also have studied lauric acid melting in a laboratory experiment. Where does the energy go, when a material is heated up such that the temperature rises? Where does the energy go when the material melts or evaporates?
When heating a material changes its temperature, the heat energy is going into increasing the atomic and molecular motion: ie. the average kinetic energy (at the molecular level) is increased. When heating a material changes its phase, the heat energy is going into breaking the attractive forces that tend to hold the molecules relatively close together: ie. the potential energy of the system is increased.
These simulations show the atomic and molecular motion that takes place at different temperatures, and the differences between some solid and liquid phases. They are provided in the form of brief, QuickTime movies.
Ice Liquid Water


Click either picture to view these molecules with an interactive Java application.
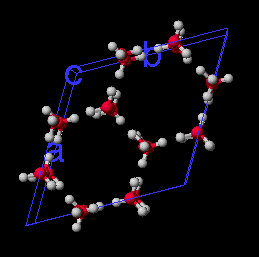
Ice I crystal. Simulation of 1 picosecond at 79 degrees K, shows individual water molecules do move, but attractive forces hold the molecules in their positions in the crystal lattice.
Ice Crystal(1M movie file)
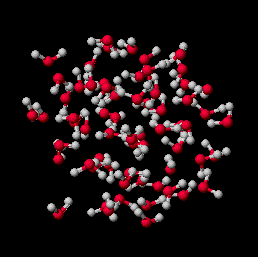
Cold water droplet. Simulation of 1 picosecond at 0 degrees C, shows individual water molecules move relative to each other and bounce off of each other, but attractive forces between one molecule and the next are sufficient to keep the water molecules from bouncing very far away.
As the water is heated, the energy goes into the kinetic energy of the molecules, which move more and more rapidly. This shows up as an increase in temperature until the water boils.
Ice Water(0.6M movie file)
Boiling water droplet. Simulation of 1 picosecond at 100 degrees C, shows that occasionally enough kinetic energy gets concentrated in an individual water molecule, that it bounces off of the others hard enough to escape from the water droplet as water vapor.
Hot Water(1.1M movie file)
Solid Lauric Acid Molten Lauric Acid
Click either picture to view these molecules with an interactive Java application.
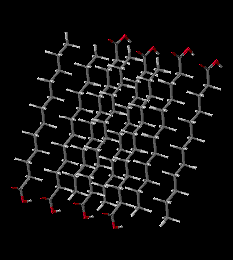
Solid Lauric Acid. Simulation of 1 picosecond at 50 degrees C, shows that the individual Lauric Acid molecules maintain their positions, packed into the semicrystalline solid. There is significant kinetic energy at this temperature, but it is mostly in the form of vibrations with movement of individual atoms, especially H-C-H bending vibrations and C-C-C-C twisting motions.
Cold Lauric Acid Solid(2.3M movie file)
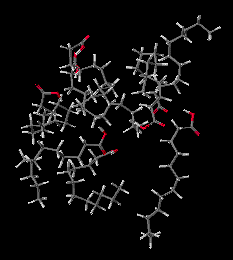
Molten Lauric Acid. Simulation of 2 picoseconds at 150 degrees C, shows that in the liquid form the Lauric Acid molecules move relative to each other. But being much larger than water molecules, it takes longer to significantly change positions.
Hot Lauric Acid(1.7M movie file)